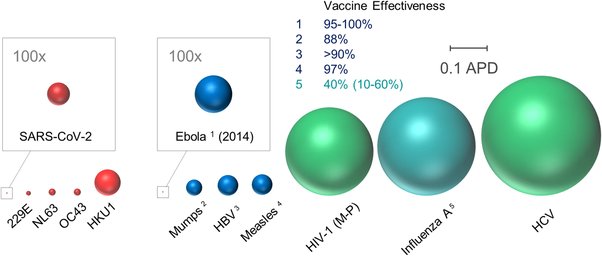
And some more encouraging news on the drug front. A bit technical, but the scale is encouraging:
Bayer, Mylan and Teva (and others): generic chloroquine
We start with the most talked-about drug, which has been around for 70 years and treats malaria: now generic chloroquine, which has been used increasingly in Asia and now in Europe to help lessen the impact of COVID-19.
In France, for instance, a professor conducted a small study of the malaria drug in 24 patients with novel coronavirus infections. Of those who received the medicine, only 25% tested positive for the virus after six days, according to en24. Meanwhile, of those who didn’t receive it, 90% tested positive after that time frame. The French government now plans to run larger studies. Bayer, Teva and Myland announced in mid-March they would be donating the drug in order for its use to be ramped up.
N.B. the above comments on chloroquine have been retained for historical reasons but that research has been repeated several times and found to be false, Chloroquine is NOT a treatment for Covid 19.
Gilead: remdesivir
This is one of the more hopeful experimental prospects. Back in February, infectious disease specialist Gilead announced it was testing the antiviral in two phase 3 trials after gaining a swift FDA IND against COVID-19. The company, which has tested the drug against other infections including SARS, MERS and most recently Ebola, is hoping it can pull out all the stops and finally see a big hit for the therapy. Trials are now ongoing, with the endpoints focused on clinical improvement.
Eli Lilly and AbCellera
Big Pharma Eli Lilly and biotech AbCellera are further back in the development path, entering the race to develop a therapy against the SARS-CoV-2 virus in March by getting hold of a blood sample from one of the first U.S. patients to recover from the pathogen.
Having secured the sample, AbCellera screened 5 million immune cells in search of ones that made the functional antibodies that helped the patient neutralize SARS-CoV-2. The screening identified 500 or so fully human antibody sequences. With the project now advancing to an assessment of the antibodies’ effectiveness against SARS-CoV-2, AbCellera has enlisted Lilly to help keep the program moving forward quickly.
Takeda: TAK-888
The Japanese Big Pharma is working on an anti-SARS-CoV-2 polyclonal hyperimmune globulin (H-IG) to treat high-risk individuals with COVID-19, which it’s calling TAK-888. H-IG is a plasma derived-therapy that has previously been shown to help those with severe acute viral respiratory infections, and it hopes it can now be a COVID-19 treatment.
“We’re collaborating with several health and regulatory agencies and health care partners across the globe to move the research forward,” it said, adding that in order to “develop TAK-888, scientists need to have access to source plasma from people who have successfully recovered from COVID-19.”
It’s also exploring whether currently marketed drugs as well as its other pipeline products could help against the virus.
Regeneron: cocktail therapy
In March, Regeneron scientists said they had isolated hundreds of neutralizing antibodies against the SARS-CoV-2 virus from a humanized mouse model as well as from humans who have recovered from COVID-19. The goal is to select the top two antibodies for a cocktail therapy, which can either be administered to at-risk people before exposure as a vaccine or as treatment for those already infected. If everything goes as planned, Regeneron aims to enter clinical studies by early summer.
All coronaviruses have a cell surface protein called the spike protein. It helps the virus bind to the host cell for an infection. Regeneron’s SARS-CoV-2 antibodies will target the spike protein in an attempt to block the interaction of the virus with the host.
Alnylam and Vir: RNAi therapeutics
Alnylam Pharmaceuticals and Vir Biotechnology teamed up in March to develop RNAi therapeutics against the coronavirus behind the COVID-19 outbreak. The collaboration builds on Alnylam’s synthesis of siRNAs that target highly conserved regions of coronavirus RNA.
Alnylam has synthesized more than 350 siRNAs targeting all available genomes of SARS-CoV-2—the virus at the heart of the outbreak—and SARS-CoV. The partners claim some of the siRNAs hit highly conserved regions of RNA, suggesting they may be broadly efficacious against coronaviruses.
Encouraged by those early data, the partners plan to put the siRNAs through in vitro potency assays. Vir will then perform in vitro and in vivo assessments of the antiviral activity of the most promising, potent prospects with a view to selecting a development candidate.
Moleculin Biotech: WP1122
Moleculin Biotech is set to test its glucose decoy prodrug WP1122 as a treatment for the novel coronavirus. The plan is underpinned by evidence the drug may starve infected cells of energy, while also exposing the COVID-19 virus to immune attacks.
Houston-based Moleculin originally identified WP1122 as a potential treatment for pancreatic cancer and glioblastoma multiforme. The plan going into 2020 was to wrap up formulation work in the first half the year and aim to file to run a clinical trial in 2021. Then the COVID-19 outbreak began, leading Moleculin to spy a new use for WP1122.
The evidence supporting the use of WP1122 against the coronavirus is early stage. Moleculin only has preclinical data to support its belief that WP1122 has better drug-like properties than 2-DG, and the case for using 2-DG against viruses other than SARS-CoV-2 is based on studies in tissue cultures and other preclinical tools.
Moleculin is working with the University of Texas Medical Branch (UTM to learn whether the early signs of promise will translate into a candidate worth testing in the clinic.
The evidence supporting the use of WP1122 against the coronavirus is early stage. Moleculin only has preclinical data to support its belief that WP1122 has better drug-like properties than 2-DG, and the case for using 2-DG against viruses other than SARS-CoV-2 is based on studies in tissue cultures and other preclinical tools. The biotech is working with the University of Texas Medical Branch (UTM to learn whether the early signs of promise will translate into a candidate worth testing in the clinic.